 ...the monthly, Open Access Publisher.
...the monthly, Open Access Publisher.
 ...the monthly, Open Access Publisher.
...the monthly, Open Access Publisher.
Research Article - (2023) Volume 14, Issue 1
Received: 08-Dec-2022, Manuscript No. IJVWS-23-93719; Editor assigned: 14-Dec-2022, Pre QC No. IJVWS-23-93719(PQ); Reviewed: 22-Dec-2022, QC No. IJVWS-23-93719; Revised: 31-Mar-2023, Manuscript No. IJVWS-23-93719(R); Published: 28-Apr-2023
Bovine brucellosis is a bacterial endemic zoonotic disease that poses a serious threat to public health, especially for the poor and marginalized communities. Between April 2021 and June 2021, a cross-sectional research of bovine brucellosis was carried out in Bero wereda, South West Ethiopia. All breeds of cattle kept in an extensive husbandry system and older than six months were selected randomly and included from the study region. A total of 111, 96, and 177 agro ecological samples from highland, lowland, and midland, respectively, were gathered based on the density of the cattle population in the wereda. All 384 sera were subjected to the screening test (Rose Bengal Plate test, or RBPT) against Brucella abortus antigen, and 13 of them tested positive for bovine brucellosis. Those RBPT positive samples were then retested by the more specific confirmatory test of indirect ELISA, of which only 11 of them were truly positive for bovine Brucella antibody, providing an overall prevalence of 2.86% with 95% CI (1.61-5.06). Age, agro ecology, and the introduction of new animals were found to have statistically significant associations (P<0.05) with the occurrence of brucellosis among the predisposing variables taken into account in the current study. Despite the low sero prevalence found in this study, anyone at risk for brucellosis should take precautions to protect themselves by using personal protective equipment, maintaining a clean barn, and disinfecting any areas that may have become contaminated by discharged material.
Brucellosis, Risk factors, Seroprevalence, Cattle, Southwest Ethiopia
Several species of the genus Brucella, primarily Brucella abortus, B. melitensis, B. suis, and B. canis, can infect both humans and animals, causing an infection known as brucellosis. Brucella abortus biovars (bv) are typically to blame for Brucella infection in cattle. B. melitensis can also cause infection if cattle are housed in close proximity to sheep or goats, mainly in southern Europe, Africa, and western Asia. The bacteria B. suis can occasionally infect cattle.
Bovine brucellosis is distinguished by abortion in pregnant cows between the fifth and ninth month of pregnancy. Adult male cattle may also develop orchitis or epididimitis, and brucellosis may result in sterility in both genderes [1].
Young animals and females who are not pregnant typically exhibit no symptoms of the illness. In some tropical areas, hygromas, which typically affect the joints of the legs, are common brucellosis presentations and may be the only outward signs of infections. Source of infection for the transmission of the bovine brucellosis are aborted fetuses, the fetal membranes after birth, and vaginal discharges and milk from infected animals. The most common routes of transmission is the gastrointestinal tract following ingestion of contaminated pasture, feed, fodder, or water, and after birth, fetuses, and newborn calves, all of which may contain large number of the organisms and constitute very important source of infection [2].
Bovine brucellosis, the most common bacterial zoonoses in the world, is also one of the most contagious and significant disease.
According to the FAO, WHO, and OIE, the disease was one of the most widespread zoonoses in the world and had a detrimental impact on public health, veterinary medicine, and the economy in developing countries. According to the World Health Organization, brucellosis is the zoonoses that most frequently affects African countries causing more disease, suffering, and economic loss than any other zoonoses. It is among the top five zoonotic diseases in Ethiopia.
Economically, bovine brucellosis results in both direct and indirect losses: Direct losses include abortion, neonatal death, replacement costs, treatment costs, labor costs, emergency slaughtering of the infected animals, and severance pay. Indirect losses include morbidity, stunting, reduced fertility, decreased milk production, decreased sale value of infected cows, lack of access to markets, restrictions on the international trade of live animals and their products, disruption of local markets, and lack of access to markets [3].
Most wealthy nations have successfully eradicated bovine brucellosis through a number of comprehensive control initiatives, but developing nations have continued to see an increase in the disease due to a lack of funding and well-coordinated control efforts. The epidemiology and preventative methods of brucellosis in humans and livestock are poorly understood in underdeveloped nations, and this is especially true in sub-Saharan Africa. Vaccination of young and mature animals and the slaughter of diseased and exposed animals, typically on the basis of a reaction to a serological test, have been the two main approaches used by control programs [4].
Numerous serological studies in Ethiopia have revealed that bovine brucellosis is an endemic and common illness in urban, peri-urban, highland and lowland, vast and intensive agricultural, small-holder farms, and ranches throughout the nation. Accordingly, 39% by Mayer in Western Ethiopia, 8.2% by Bayleyegne in Central Ethiopia, 22% by Tariku in Northeastern Ethiopia, 8.1% by Yilkal in Addis Ababa and the surrounding area, 11.15% by in South eastern Ethiopian dairy farms and ranches, 7.7% by in Tigray region, 0.14% by in north Gonder zone, 0.77% by in southwestern Ethiopia, 1.11% by in Addis Ababa and Sululta abattoir, 2.46% by in Sidama zone of southern Ethiopia, 22% by Sintaro (20) in dairy herd of Cheffa state farm, and 5% by in cattle under crop-livestock mixed farming were documented respectively. Although much work has been done and reports are available in Ethiopia, there is no information on the status of bovine brucellosis in South West Ethiopia regional state of the country. Only fragments of information are available from regional Agricultural Development Bureau that the disease is recorded in all zones in the regions with apparently low incidence. Therefore the general objectives of this cross-sectional study were:
• To determine the sero-prevalence of bovine brucellosis in Bero wereda.
• To identify risk factors and quantify their degree of association with brucellosis in cattle.
The study area
A cross-sectional study on bovine brucellosis was conducted in Bero wereda, one of the wereda of the Southwestern Ethiopia Regional State of Ethiopia, part of the West Omo Zone, from April 2021 and June 2021. The Surma wereda wereda on the south, the Gambela regional state on the west, the Guraferda wereda on the north, the Gorigesha wereda on the northeast, and the Maji wereda on the east are its interfaces. The wereda's exact location may be found at 06°24′ North latitude and 35°15′ East longitude, with an altitude range of 691–1736 masl. The annual average temperature in the wereda ranges from 18.1 to 40 degrees, and the region gets 600 to 900 mm of rainfall annually (Figure 1) [5].
Study design
To determine the sero-prevalence of bovine brucellosis and associated risk variables in the study sites, a cross-sectional study design was used.2.3.
Study population
Randomly chosen from the research region were cattle of all breeds that were 6 months of age and older and housed in an extensive husbandry system. For the study, individuals of both genderes and those older than six months were chosen. Age was assessed, according to Pace and by examining the owners' lower incisor teeth and asking them questions about the lives of their cattle’s. The animals were then divided into three age groups: young (<3 years), adult (3-6 years), and old (>6 years) [6].
Sampling technique and sample size determination
Both randomized and purposive sampling techniques were employed to select the study sites and study animals (cattle).
Households and study units/individual cattle were selected using a simple random sample technique, whereas study wereda and kebeles were relied on reports of syndromic outbreak incidence of brucellosis.
Because no earlier studies on the bovine brucellosis in cattle identified in Bero wereda had been conducted, the current study took into account the 50% predicted prevalence, the 95% confidence level, and the 5% absolute precision or marginal error. The number of animals needed for the investigation was calculated using the formula based on these hypotheses [7].
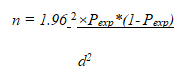
Where n=required sample size, d=desired absolute precision, and Pexp=expected prevalence (50%) Based on the formula, the total sample size was computed to be 384 cattle to be selected from wereda. Based on density of cattle population in the wereda, a total of 111, 96, and 177 samples were collected agro ecologically from highland, lowland, and midland, respectively.
Methodology
An average of 8 ml-10 ml whole blood was drawn from jugular vein of each 384 cattle into labeled plain vacutainer test tubes using 18 gauge needles. Sera were separated from the blood into labeled cryogenic vials. The serum was placed into portable fridge until its delivery to the laboratory for investigation. In the laboratory all serum sample were stored at -20°C as to achieve very good preservation prior to serological analysis.
Screening test by Rose Bengal Plate Test (RBPT)
Each serum sample was tested against Brucella agglutinin antigen using sensitive RBPT technique based on the protocol of the OIE.
Confirmatory test by indirect ELISA
According to the OIE's recommended protocols(33), additional testing was done on those sera that RBPT clearly identified as positive using the more precise confirmatory indirect ELISA in order to identify the existence of anti-Brucella antibodies in the sera.
Description and principle of indirect ELISA (IDvet, France): Wells are coated with purified Brucella abortus LPS. Specimens to be tested and the controls are added to the micro wells diluted at 1/20. Anti-Brucella antibodies, if present form an antibody-antigen complex. A multi-species Horse Radish Peroxidase (HRP) conjugate is added to the micro wells. It fixes to the anti-Brucella antibodies, forming an antigen- antibody- conjugate –HRP complex.
After washing in order to eliminate the excess conjugate, the substrate solution is added. The resulting coloration depends on the quality of specific antibodies present in the specimen to be tested.
In the presence of antibodies, blue solution appears which becomes yellow after addition of the stop solution. In the absence of antibodies, no coloration appears. The microplate is read at 450 nm.
Questionnaire survey
Semi structured questionnaire were administered to selected cattle owners following verbal consent on the need of the study. For each animal sampled, questionnaire data were collected concerning age, gender, introduction of new animal, body condition and study site (agro ecology) to analyze the impact of these variables on the occurrence of the disease.
Data management and analysis
Relevant information was coded, organized, and entered into a Microsoft Excel sheet. Organized data were transfer to SPSSV.20 for analysis. During data analysis, descriptive and logistic regression analyses were used. To find the disease's prevalence and other frequencies, descriptive statistics will be used. The strength of the association between those contributing factors and the disease was calculated using binary and multinomial logistic regression analysis. The total number of animals required for the investigation was determined using the Thrusfield formula.
Age, gender, the introduction of new cattle, the size of the herd, and the study site (agro ecology) were the associated variables that were used to determine prevalence and percentages in connection with the findings of the confirmatory tests, as summarized in Table 1. 384 different animals were sampled in total (260 females and 124 males) [8].
All 384 sera were subjected to the screening test (Rose Bengal Plate test-RBPT) against Brucella abortus antigen, and 13 of them have come positive for bovine brucellosis with an overall prevalence of 3.38% with 95% CI (1.99–5.71).
Those RBPT positive samples were further retested by the more specific confirmatory test of indirect ELISA of which only 11 of them were real positive for bovine Brucella antibody providing an overall prevalence of 2.86% with 95% CI (1.61–5.06).
| Variable | Category | Sample examined | Number of positive | Sero prevalence (%) ( 95% CI) |
|---|---|---|---|---|
| Agro ecology | Highland | 111 | 2 | 1.8 (0.5-6.33 ) |
| Lowland | 96 | 7 | 7.29 (3.58-14.29) | |
| Midland | 177 | 2 | 1.13 (0.31-4.03) | |
| Age | <3 year | 81 | 1 | 1.23 (0.22-6.67) |
| 3-6 year | 217 | 3 | 1.38 (0.47-3.99) | |
| >6 year | 86 | 7 | 8.14 (4-15.86) | |
| Gender | Male | 124 | 1 | 0.81 (0.14-4.43) |
| Female | 260 | 10 | 3.85 (2.1-6.93) |
Logistic regression: Binary and multinomial logistic regressions were conducted to determine the effect of associated studies on the determinants of the disease. Unadjusted odds ratios were calculated using binary logistic regression separately for each factor (Gender, herd size, age, agro ecology, and introduction of additional animals) in order to determine the potential impact of each factor on the disease using 95% CI and P<0.05 (Table 2). Similarly, adjusted odds ratio (AOR) was calculated concurrently to estimate the true impact of factor one (without compounding effect) on the disease [9].
| Variable | Category | Total examined |
Positive | COR(95%CI) | P-value | AOR(95%CI) | P-value |
|---|---|---|---|---|---|---|---|
| age | <3 years | 81 | 1 | - | 0.014 | - | - |
| 3-6 years | 217 | 3 | 1.12 (0.11-10.9) | 0.921 | 5.4 (0.56-54) | 0.16 | |
| >6 years | 86 | 7 | 7.09 (0.85-58.9) | 0.07 | 8.4 (1.6-43) | 0.012 | |
| Gender | Male | 124 | 1 | - | - | - | - |
| Female | 260 | 10 | 4.9 (0.623-38.8) | 0.131 | 3.54 (0.4-31.7) | 0.26 | |
| Herd size | Small | 163 | 1 | - | - | - | - |
| Large | 221 | 10 | 7.7 (0.97-60.6) | 0.053 | 2.8 (0.3-26.8) | 0.36 | |
| Introduction of | Yes | 55 | 5 | 5.4 (1.5-18) | 0.007 | 8.1 (1.8-36.2) | 0.006 |
| new animal | No | 329 | 6 | - | - | - | - |
| Agro ecology | High land | 111 | 2 | - | 0.027 | - | - |
| low land | 96 | 7 | 4.3 (0.87-21.15) | 0.074 | 10.8 (1.6-71.7) | 0.014 | |
| Mid land | 177 | 2 | 0.62 (0.09-4.5) | 0.638 | 3.77 (0.6-22) | 0.14 |
In the current investigation, Brucella antibody determination was carried out using the Rose Bengal Plate Test (RBPT) and the Indirect Enyme-Linked Immunosorbent Assay (I-ELISA). Accordingly, 2.86% true seroprevalence of Brucella antibodies was detected at the animal level by an indirect ELISA test [10]. To increase test result accuracy and adopt the most common testing strategy, epidemiological investigations should serially administer two tests. Indirect ELISA is employed as a confirmatory and screening test for the identification of Brucella antibodies in the diagnosis of bovine brucellosis, whereas RBPT is a very sensitive test that may readily be applied in field conditions for the screening purpose. Cross-reactions with other bacteria's. Smooth Lipopolysaccharide (S-LPS) antigens may cause two false positive serological responses in RBPT [11]. In this situation, seropositivity was only the result of a natural infection because there has never been a history of vaccination in the study population. This relatively low prevalence of the diseases might be attributable to keeping of cattle in individual grazing land in the highland area and extensive grazing conditions in lowland area of the wereda could reduce both animal to animal contact and contamination of pasture under dry climatic conditions. Another reason could be that, in the area studied, the mixing of cattle from many herds, especially at grazing and watering points is less marked than in the pastoral livestock production system [12].
Comparable studies have been reported by deferent scholars in deferent regions of Ethiopia. A 2.9% by in central Oromia, A 3.1% seroprevlence of bovine brucellosis in Jimma zone of Oromia region. 3.65% in and around Waliso town, Oromia region, 3.19% in the extensive production of system of Tigray region(28) and 3.5% traditional livestock husbandry practice in southern and Eastern Ethiopia [13]. The present study has revealed higher prevalence than some previous studies of bovine brucellosis in Ethiopia. Studies lower than the present report included 1.92% % prevalence reported from Sidama zone 1.97% by in Guto- Gida district of East Wollega Zone, 0.77% by in Jimma zone, western Ethiopia, 1.2% by in Tigray region, and 0.14% in North Gonder one On the other hand,, the sero prevalence reported in the current study was lower than the values 4.9% in western Tigray 11% in Wuchale Jida wereda, central Ethiopia 4.3% in Adami Tulu, Central Ethiopia14.1% in Assela 10.6% in Borena.
Similarly relatively higher sero-prevalence was reported in other African countries. For instance, 24.5% in Sudan, 24% from Nigeria, 6.6% in Chad, 6.6% in Ghana and 46.8% in Uganda. Various factors, such as varied animal management practices, age, gender, geographical deference, reproductive diseases, herd size, sample size, and the serological tests used that would further emphasize these differences, could be responsible for the variation in sero prevalence of bovine brucellosis among different regions in Ethiopia [14].
Age, agro-ecology, and the introduction of new animals were noticed to have statistically significant correlations (P<0.05) with the occurrence of brucellosis, whereas the gender of the animals and the size of the herd could do not (P>0.05). These associations were among the associated factors that should be considered in the present study [15].
In this study, older animals beyond six years of age were 7 times (OR =7.09 (95% CI 0.85-58.9) more likely to contract the infection than their younger counterparts. Significantly higher sero-prevalence was seen in the older age category than the younger age category. This finding concurs with that of who found that 52% of the sero positive cows were older than 6 years. According to reports, the age of the individual cattle can affect their susceptibility to infection by. Although latent infections often occur, younger animals tend to be more resistant to infection and commonly clear infections [16].
The sero prevalence of newly introduced animals was high, and significantly associated with the occurrence of bovine brucellosis in the study wereda.
Newly introduced animals were five times COR 5.4 (1.5-18) more exposed to bovine brucellosis than animals reared within house. This might be due to the new animals purchased from bovine brucellosis infected area. During a questionnaire interview majority of the new animals purchased from pastoral weredas of Surma and Maji. Accordingly Negash and Dubie the prevalence of bovine brucellosis in pastoral area were high when compared to small holder farmers, this is due to the pastoralists transhumant way of life leads keeping and mixing of many herds together, which assist the transmission of brucellosis infection among herds [17].
The present study also revealed that the seroprevalence of bovine brucellosis was significantly associated within the agro ecology of the study kebeles. High seroprevalence of bovine brucellosis found in lowland (<1500 masl) area 7.33% with (95% CI 3.58-14.29%) of the wereda. This could be due to availability of common grazing land in lowland area, but in the high land area majority of the house hold kept by tieing their cattle’s in their own individual grazing area, so there would be high contamination of Brucella infection by herds during grazing in the lowland area [18].
This study found that there had been a low record of bovine brucellosis in the Bero wereda. The disease's important risk factors now include aging, agro ecology, and the addition of new animals. Developing effective control techniques and increasing public awareness of brucellosis zoonotic transmission are therefore necessary. Furthermore, more research is required to identify and characterize how brucellosis causes problems with reproduction and the consequent decrease in the studied areas.
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Select your language of interest to view the total content in your interested language